PCT (procalcitonin)
A biomarker to aid in the diagnosis of sepsis
-
 Fast results
Fast results
-
 Early detection of sepsis
Early detection of sepsis
-
 Better monitoring of disease progression
Better monitoring of disease progression
PCT testing in the emergency department
Many patients arrive at the emergency department with a local infection or, in some cases, develop an infection after major trauma or surgery.
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. Sepsis is the number 1 cause of death in hospitals [2].
Sepsis can be difficult to identify, particularly in the initial stages, and it presents a considerable diagnostic challenge to the emergency department and intensive care clinicians.
Procalcitonin (PCT) is released in larger amounts in response to infection, thereby acting as a risk indicator for sepsis.
Immunoassay products and solutions

PCT - a biomarker to aid in the diagnosis of sepsis
PCT is synthesised primarily by the C-cells of the thyroid gland, and to a lesser extent in the neuroendocrine tissue of other organs such as the lungs and intestines. Normal procalcitonin levels in the blood are very low [3].
However, production can be stimulated in almost every organ by inflammatory cytokines and especially bacterial endotoxins present e.g., during sepsis, causing high amounts of PCT to be released in the blood. This allows PCT levels to be used as a biomarker of sepsis. The higher the level of PCT, the greater the likelihood of systemic infection and sepsis [3].
The added value of point-of-care testing
The mortality rates associated with sepsis remains higher than prostate cancer, breast cancer, and HIV/AIDS combined [4], despite strict guidelines for the implementation of early and effective therapies which have improved the chance of survival [5].
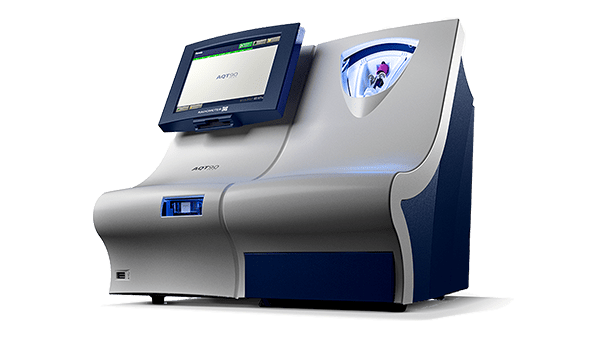
Radiometer’s rapid, acute care AQT90 FLEX PCT test, an in vitro diagnostic assay for the quantitative determination of PCT in EDTA or lithium-heparin whole blood or plasma specimens, is an important tool for acute and intensive care professionals, aiding the early detection of sepsis. This enables clinicians to start antibiotic treatment undelayed.
PCT has the potential advantage of earlier disease diagnosis and better monitoring of disease progression. Early diagnosis and treatment are critical for survival rates [6].
Testing PCT on the AQT90 FLEX analyser
Radiometer’s AQT90 FLEX immunoassay analyser is a benchtop analyser that brings rapid biomarker assessment capabilities right to the patient’s bedside. The AQT90 FLEX delivers PCT results in less than 21 minutes, aiding the clinicians in the work-up of critically ill patients at risk for sepsis [7].
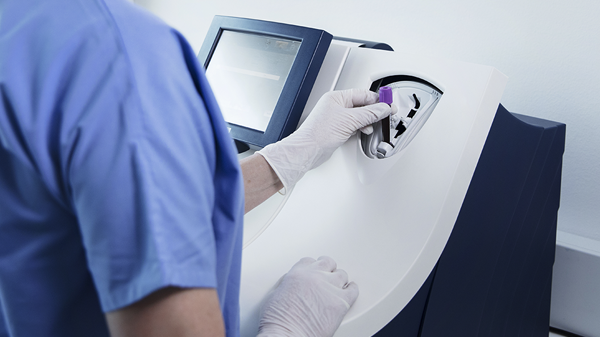
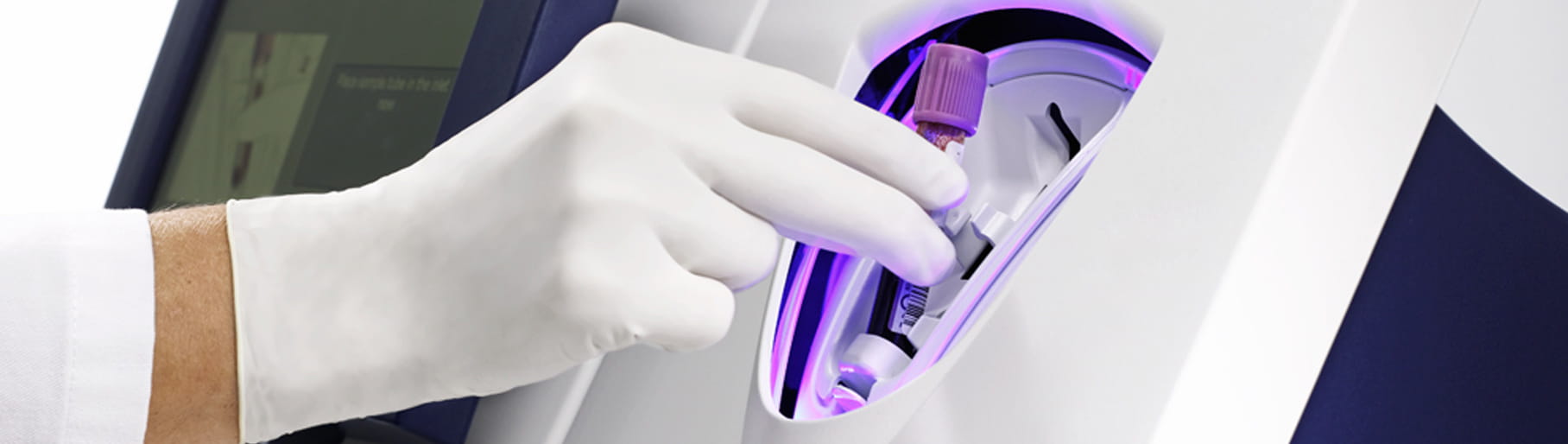
Its closed tube system makes PCT testing easy: the operator simply inserts the sample tube into the tube holder in the sample port and the analyser performs all assay steps automatically. There is no need for sample preparation.
PCT assay specifics
References
2. Rudd et al. Global, regional, and national sepsis incidence and mortality, 1990 -2017: analysis for the Global Burden of Disease Study. The Lancet Volume 395, Issue 10219, 18 -24 January 2020; 200-211.
3. Sartelli M, et al. The role of procalcitonin inreducing antibiotics across the surgical pathway. World Journal of Emergency Surgery. 2021
4. Vincent JL. Increasing awareness of sepsis: World Sepsis Day. Crit Care 2012
5. Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010
6. Riedel S, et al. Procalcitonin as a Marker for the Detection of Bacteremia and Sepsis in the Emergency Department. Am J Clin Pathol 2011
7. Li et al. Determining procalcitonin at point-of-care; A method comparison study of four commercial PCT assays. Practical Laboratory Medicine 2021; 25.
Cookies are used on this website
Use of cookiesPlease enter a valid email
We will be sending an e-mail invitation to you shortly to sign in using Microsoft Azure AD.
It seems that your e-mail is not registered with us
Please click "Get started" in the e-mail to complete the registration process
Radiometer is using Microsoft AZURE Active Directory to authenticate users
Radiometer uses Azure AD to provide our customers and partners secure access to documents, resources, and other services on our customer portal.
If your organization is already using Azure AD you can use the same credentials to access Radiometer's customer portal.
Key benefits
- Allow the use of existing Active Directory credentials
- Single-sign on experience
- Use same credentials to access future services
Request access
You will receive an invitation to access our services via e-mail when your request has been approved.
When you accept the invitation, and your organization is already using AZURE AD, you can use the same credentials to access Radiometer's customer portal. Otherwise, a one-time password will be sent via e-mail to sign in.