Lactate as an aid in sepsis diagnosis and management
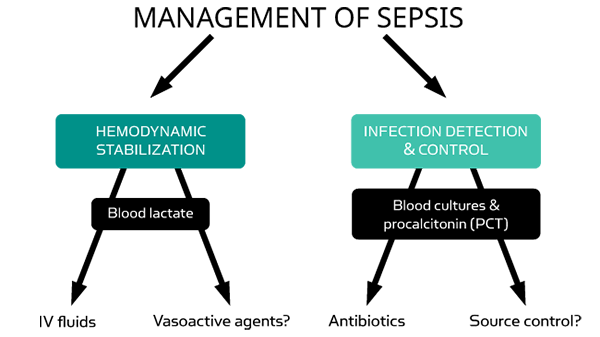
Lactate and PCT are complementary markers to aid in the diagnosis and management of sepsis and septic shock.
- Sepsis: Sepsis should be defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Organ dysfunction can be represented by an increase in the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score of 2 points or more [1].
- Septic shock: Septic shock should be defined as a subset of sepsis in which
particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. Patients with septic shock can be clinically identified by a vasopressor requirement to maintain a mean arterial pressure (MAP) ≥ 65mmHg and lactate > 2 mmol/L (>18mg/dL) in the absence of hypovolemia [1].
PCT is typically the primary biomarker to aid in detection or rule-out of early sepsis
When measuring lactate in the ED setting the results are complementary to other test results, such as PCT for determination of level of bacterial or fungal infection, and together they serve as an important help in assessing the severity of the illness [3].
Lactate levels should be measured within 3 hours of admission and if elevated repeated within 6 hours, as recommended by the Surviving Sepsis Campaign guidelines [4].
This allows for the implementation and evaluation of effective hemodynamic management of the septic patient as early as possible, increasing the chances of survival [1, 3-5].
ss
Detecting sepsis
A simple infection can rapidly develop into sepsis a life-threatening condition which requires on-the-spot diagnosis and treatment while the condition is still in its early stages [1].
Diagnosing patients suspected of sepsis is both challenging and complex. Often patients arriving at the ED are febrile and have shortness of breath, low blood pressure, a high pulse and respiration - symptoms which can easily be confused with other severe conditions [3].
Early diagnosis and immediate treatment are crucial to increase the chances of surviving sepsis. The clinical evaluation alone is often insufficient for an early diagnosis of sepsis [5].
PCT and lactate are useful as aids in the diagnosis and management of sepsis, and complement the general clinical assessment of the patient’s physical condition [3].
What is lactate?
Lactate is a metabolite of glucose produced by tissues in the body under conditions of insufficient oxygen supply. Lactate is normally cleared by the liver and the kidneys, and the blood lactate concentration in unstressed patients is 1-1.5 mmol/L [6].
Elevated lactate levels indicate an imbalance and are associated with increased mortality in sepsis [5,6,7].
The value of measuring lactate in the diagnosis of sepsis
Patients with elevated lactate levels are seriously ill and require effective treatment here and now. Measuring lactate levels provides useful information about the progression of the condition and the effectiveness of the treatment [3].
For patients already suspected of sepsis, measuring the lactate levels provides useful information on the severity of the condition and enables monitoring of disease progression [3].
Find out more about cost-efficient lactate analysis or the role of PCT as an aid in the diagnosis of sepsis.
Visit acutecaretesting.org for more in-depth articles on diagnosing sepsis.
References
1. Singer M, Deutschman CS, Seymour CW et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315,8: 801-10
2. Vijayan AL, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. Journal of Intensive Care 2017
3. Freund Y et al. Serum lactate and procalcitonin measurements in emergency room for the diagnosis and risk-stratification of patients with suspected infection. Biomarkers 2012; 17 (7): 590-596
4. Dellinger RP, Levy MM, Rhodes A et al. Surviving sepsis campaign. International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41: 580-637
5. Riedel S, et al. Procalcitonin as a marker for the detection of bacteremia and sepsis in the emergency department. Am J Clin Pathol 2011; 135:182-89
6. Seeger C et al. Acute care testing handbook. 2014
7. Andersen LW, et al. Etiology and therapeutic approach to elevated lactate. Mayo Clin Proc. 2013; 88(10): 1127-1140
Cookies are used on this website
Use of cookiesPlease enter a valid email
We will be sending an e-mail invitation to you shortly to sign in using Microsoft Azure AD.
It seems that your e-mail is not registered with us
Please click "Get started" in the e-mail to complete the registration process
Radiometer is using Microsoft AZURE Active Directory to authenticate users
Radiometer uses Azure AD to provide our customers and partners secure access to documents, resources, and other services on our customer portal.
If your organization is already using Azure AD you can use the same credentials to access Radiometer's customer portal.
Key benefits
- Allow the use of existing Active Directory credentials
- Single-sign on experience
- Use same credentials to access future services
Request access
You will receive an invitation to access our services via e-mail when your request has been approved.
When you accept the invitation, and your organization is already using AZURE AD, you can use the same credentials to access Radiometer's customer portal. Otherwise, a one-time password will be sent via e-mail to sign in.